The Use of Natural Products to Destabilise Biofilms?
There is relatively little information on specific control of Campylobacter biofilms, but more research has been done in the control of biofilms in other areas (such as the dental area) and a broad range of natural products are known to destabilise or reduce biofilms, herbal products such as Trigonella (Seed) Extract, [1] Chamaemelum Extract, [2] Tannins, [3] Thyme and Olive leaf extracts, [3] diallyl-sulphide, [5] bacteriophages, furanones that interfere with quorum sensing molecules, [6] bacteriocins, [7,8] a broad range of peptides, [9,10,11] alkaloids, [12] non-cariogenic sugars (Tagatose, [13] Xylitol [14]) and glycolipids. [15] DNA, proteins, and EPS constitute the biofilm matrix, and recent studies revealed that effective disruption of the biofilm architecture could be achieved by various enzymes. Matrix-degrading enzymes such as DNase, alginate lyase, [16] amylase [17] lactonase, [18] glucan hydrolase, [19] have all played a role in the degradation of biofilms from a range of different bacteria.
This feasibility project is expected to investigate the use of food-grade enzymes to specifically degrade Campylobacter biofilms, and examine the possible synergy of the approach with natural-food grade herbal extracts or actives. It will also determine if suitable grafting/coating of enzymes and herbal actives in food-packaging could allow the control of Campylobacter, both in the packaging itself and in poultry meat.
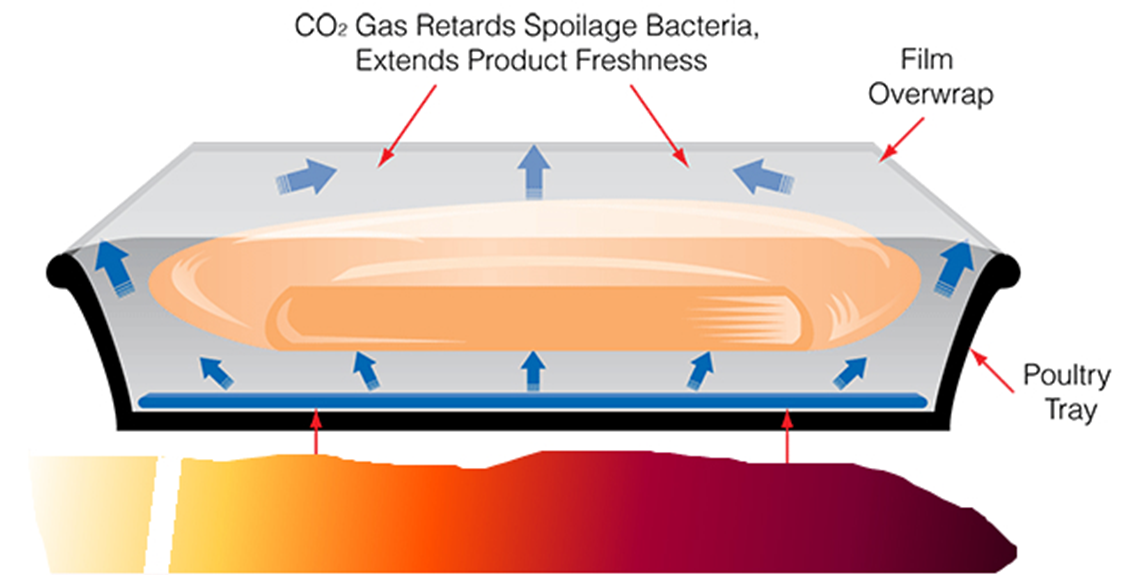 Figure 5- Model packaging to be developed in subsequent projects
Figure 5- Model packaging to be developed in subsequent projects
Photo credit: Genuineideas.com
In a subsequent stage of packaging development, the data obtain in the present feasibility will feed into a design of a new anti-biofilm coating.
At this stage it is expected that such coating will impart multiple anti-Campylobacter effects (see Figure 5),
a) on the interface meat- packaging it will interact via enzymes grafted on the plastic that will cleave selectively the Campylobacter biofilm, while
b) the herbal extract release will act synergistically to improve the control of the pathogen.
References
- Husain FM, Ahmad I, Khan MS, Al-Shabib NA., Trigonella foenum-graceum (Seed) Extract Interferes with Quorum Sensing Regulated Traits and Biofilm Formation in the Strains of Pseudomonas aeruginosa and Aeromonas hydrophila. Evid Based Complement Alternat Med. 2015; 879540.
- Kazemian H, Ghafourian S, Heidari H, Amiri P, Yamchi JK, Shavalipour A, Houri H, Maleki A, Sadeghifard N. Antibacterial, anti-swarming and anti-biofilm formation activities of Chamaemelum nobile against Pseudomonas aeruginosa. Rev Soc Bras Med Trop. 2015 Aug; 48(4):432-6.
- Castillo S, Heredia N, García S., 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol (Praha). 2015 Jan; 60(1):89-95.
- Pogačar MŠ, Klančnik A, Bucar F, Langerholc T, Možina SS.Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene& intestine epithelial cells. J Sci Food Agric. 2015 Aug 25.
- Lu X, Samuelson DR, Rasco BA, Konkel ME. Antimicrobial effect of diallyl sulphide on Campylobacter jejuni biofilms.J Antimicrob Chemother. 2012 Aug; 67(8):1915-26.
- Castillo S, Heredia N, García S., 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol (Praha). 2015 Jan; 60(1):89-95.
- Field D, Gaudin N, Lyons F, O'Connor PM, Cotter PD, Hill C, Ross RP. A bioengineered nisin derivative to control biofilms of Staphylococcus pseudintermedius. PLoS One. 2015 Mar 19; 10(3):e0119684.
- Chopra L, Singh G, Kumar Jena K, Sahoo DK. Sonorensin: A new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep. 2015 Aug 21;5:13412
- Sánchez-Gómez S, Ferrer-Espada R, Stewart PS, Pitts B, Lohner
K, Martínez de Tejada G.Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol. 2015 Jul 7;15:137. - Mishra B, Lushnikova T, Wang G. Small lipopeptides possess anti-biofilm capability comparable to daptomycin and vancomycin. RSC Adv. 2015; 5(73):59758-59769.
- Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock RE. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother. 2014 Sep; 58(9):5363-71.
- Kumar P, Selvi SS, Govindaraju M.In vitro anti-biofilm and anti-bacterial activity of Junceella juncea for its biomedical application. Asian Pac J Trop Biomed. 2012 Dec;2(12):930-5.
Rane RA, Karpoormath R, Naphade SS, Bangalore P, Shaikh M,
Hampannavar G.Novel synthetic organic compounds inspired from antifeedant marine alkaloids as potent bacterial biofilm inhibitors. Bioorg Chem. 2015 Aug; 61:66-73.
Si X(1), Quan X, Wu Y.A small-molecule norspermidine and norspermidine-hosting polyelectrolyte coatings inhibit biofilm formation by multi-species wastewater culture. Appl Microbiol Biotechnol. 2015 Sep 9. - Patent WO2003059359A1 - D-tagatose as an anti-biofilm agent
- Burt BA. 2006. The use of sorbitol and xylitol sweetened chewing gum in control. J Am Dent Assoc 137:190–6.
- Inès M, Dhouha G.Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr Res. 2015 Jul 31;416:59-69.
- Alkawash MA, Soothill JS, Schiller NL. 2006. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Acta Pathol Microbiol Immunol Scand 114:131–8.
- Craigen B, Dashiff A, Kadouri DE. 2011. The use of commercially available alpha-amylase compounds to inhibit and remove Staphylococcus aureus biofilms. Open Microbiol J 5:21–31.
- Kiran GS, Sabarathnam B, Selvin J. 2010. Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei. FEMS Immunol Med Microbiol 59:432–8.
- Pleszczyńska M, Wiater A, Janczarek M, Szczodrak J. (1→3)-α-d-Glucan hydrolases in dental biofilm prevention and control: A review. Int J Biol Macromol. 2015 Aug; 79:761-78.
